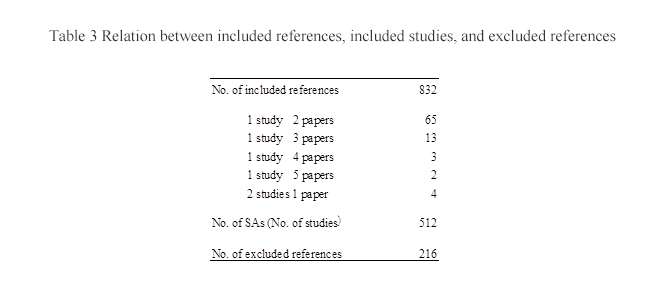
(1) Relations among included references, included studies, and excluded references
SAs were prepared for the 832 selected references including 65 cases of “2 papers for 1 study,” 13 cases of “3 papers for 1 study,” 3 cases of “4 papers for 1 study,” 2 cases of “5 papers for 1 study,” and also 4 cases of “1 paper for 2 studies,” and resulted in the preparation of 512 SAs (corresponding to 502 RCTs and ten meta-analyses). The number of excluded references deviating from the inclusion criteria was 216 (Table 3).
The International Committee of Medical Journal Editors (ICMJE) developed the manuscript guidelines initially called “Uniform Requirements for Manuscripts Submitted to Biomedical Journals (URM)” in 1979, which were updated and renamed in accord with ICMJE Recommendations in 2013, with subsequent revisions almost annually.
https://www.icmje.org/recommendations/archives/
Worldwide, more than 5000 journals follow the ICMJE Recommendations.
https://www.icmje.org/journals-following-the-icmje-recommendations/
The revisions in and after 1984 mention duplicate publication and require that submission of already published study contents be approved by the editorial committee. The ICMJE Recommendations permits secondary publication for the following cases: editors of both journals concerned have accepted; the second publication targets different readers from those of the first publication; the second publication faithfully reflects the data and interpretations in the first publication; and the second publication specifies that it is a “secondary publication.”
As shown in Table 3, it was unclear whether the considerable number of duplicate publications about clinical studies on Kampo medicines were secondary publications or versions. Submitting already published contents without permission of the editorial committee constitutes not only ethical but also copyright issues. With rising interests in publication-related ethics at home and abroad, consideration should be given to duplicate publication in papers on Kampo as well. These findings were published as study results in the following reference:
In the following symposium, the issue of duplicate publication was addressed from the perspective of the above study:
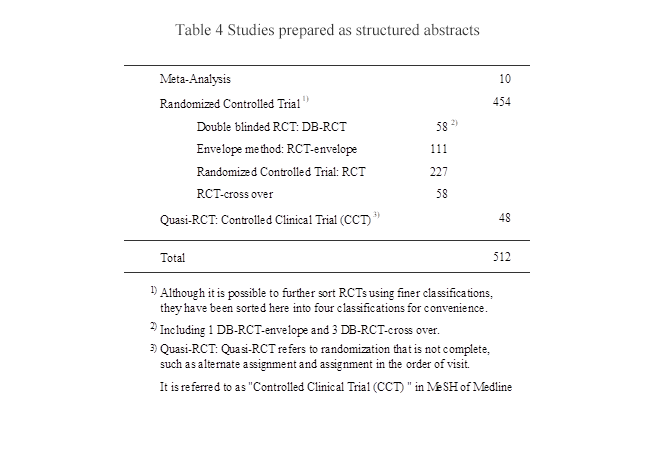
(2) Studies compiled as structured abstracts
For the studies shown in Table 4, structured abstracts were prepared.
For studies compiled as structured abstracts, the following items were indicated in the structured abstract and included the reference list: 1) SA No.; 2) ICD10 (2003 revision) code of disease; 3) research question; 4) name of Kampo formula; 5) bibliographic items of the reference; 6) study design; and 7) search source.
Research questions were supposed to be formulated with four items of patient, intervention, control, and outcome (PICO), but were simplified here.
(3) Preparation of excluded references list
The references not compiled as structured abstracts but listed as excluded references along with bibliographic items and the reason for exclusion were:
- 1) Clinical articles but not RCTs or meta-analyses
- 2) Those using formulations not approved for manufacture and sale in Japan as Kampo extract formulations (ex. Kampo decoctions, Chinese formulations)
- 3) Those using Kampo formulations in or before 1985 (with different quality from the current standards)
- 4) Citations of existing RCT articles.
- 5) Indicated with insufficient clarity to prepare the structured abstract
- 6) Others
Furthermore, when the SA of a brief report or news article was prepared and subsequently its final report was collected, then the previous SA and its source report or article were removed because of “6) Others” above, and transferred to the list of excluded references with statement of justification. Although EKAT initially emphasized comprehensiveness and contained a broad range of articles including medical society interview columns, this led to issues such as sample size or result inconsistencies between earlier and final reports of the same study, or to link errors between the RCTs and their reports. For this reason, starting with EKAT Appendix 2014, brief reports or news articles obviously written by study report authors are excluded, and previously prepared SAs are reviewed upon collection of the final reports. Consequently, one report in EKAT Appendix 2014 and two reports in EKAT Appendix 2015 have been transferred to the list of excluded references.
Finally, 216 references appear on the excluded references list.
